ISO14708-1 Defibrillator Test Pulse Generator
Defibrillator Test Pulse Generator
- Model:
Defibrillator Test Pulse Generator

Product Overview
The KP-1050S defibrillation test pulse generator is used to evaluate whether active implantable medical devices will be permanently affected when subjected to damped sine defibrillation waveforms and truncated exponential defibrillation waveforms , whether they can be reprogrammed to restore settings , and to evaluate the safety and programmability of the product. The KP-1050S defibrillation test pulse generator complies with ISO14708-1 and GB 16174.1-2024 20.2 standards. The following test procedures are included:
Test 1
Test Purpose :
To evaluate whether active implantable medical devices are permanently affected when subjected to damped sinusoidal defibrillation waveforms and whether they can be reprogrammed to restore settings.
Test equipment :
· Defibrillation test voltage generator, used to provide a damped sinusoidal defibrillation waveform with specific characteristics.
Test parameters :
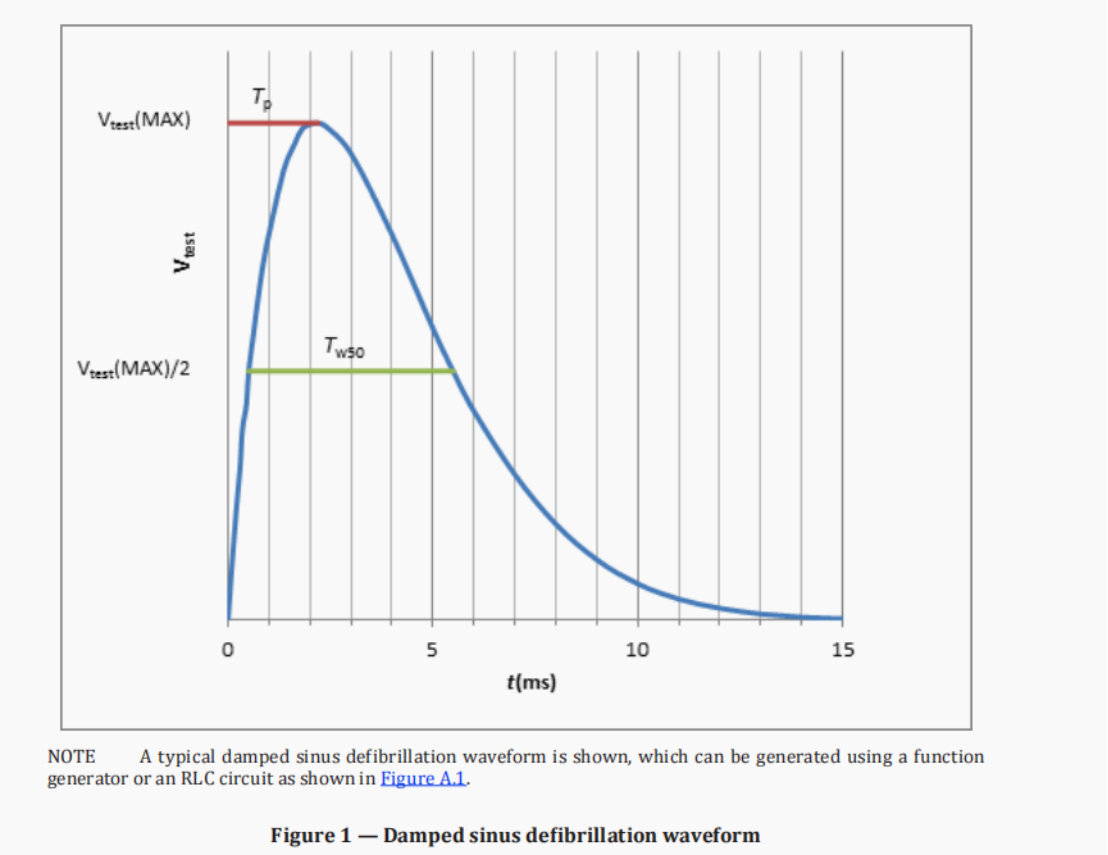
· The parameters of the damped sinusoidal defibrillation waveform must meet the following requirements: 1.5ms < Tp < 2.5ms; 3ms < Tw50 < 5.5ms; where Tp is the time interval from the start of the waveform to the maximum value, and Tw50 is the time interval above 50% of the maximum value.
· The maximum value of the test voltage Vtest is adjusted to 140+70V.
Test waveform:
Test 2
Purpose of the study :
To further evaluate the safety and reprogrammability of active implantable medical devices when subjected to truncated exponential defibrillation waveforms.
Test equipment :
· A defibrillation pulse generator is used to provide a truncated exponential defibrillation waveform of a specific duration.
Test arrangement :
· Test arrangement using C1=(150±50)μF, R1=65Ω and 2 sets of coupled switches S1 and S2.
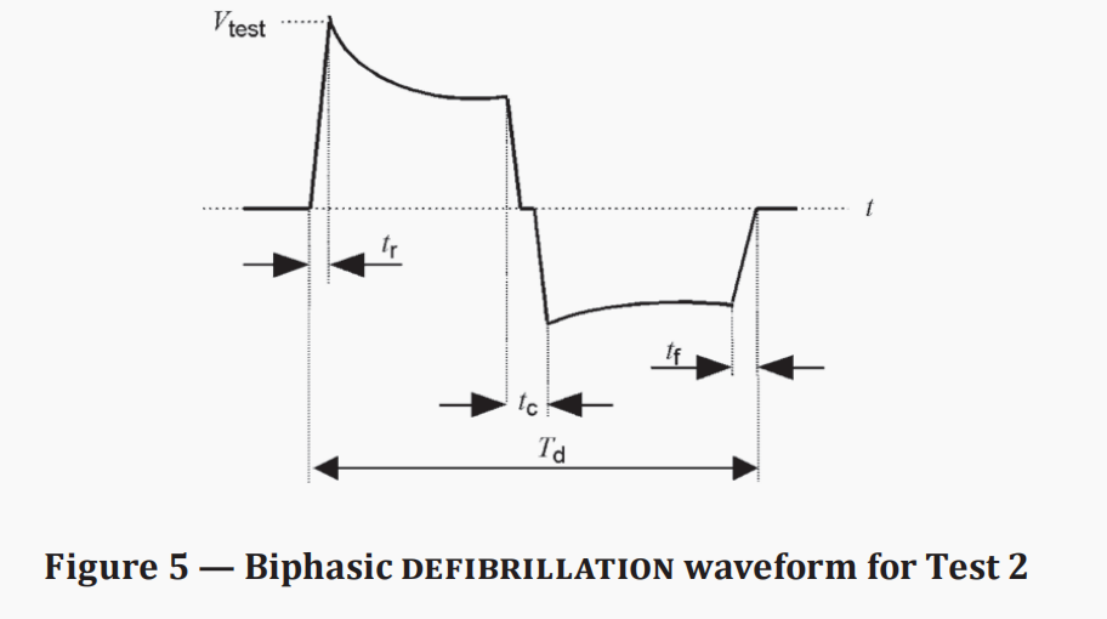
· A single-phase or bi-phase truncated exponential defibrillation waveform with a duration of Td = (10 ± 0.5) ms is generated between output terminals A and B.
Test parameters :
· The test voltage-time parameters of the truncated exponential defibrillation waveform must meet the numerical range in Table 1.
· The maximum value of the test voltage Vtest is adjusted to 140+70V.
Test waveform:
This model of pulse tester uses a programmable controller as its core and is equipped with a color touch screen for intuitively displaying and setting all working parameters and instrument status. Its user-friendly design makes the operation interface concise and clear, and it can memorize various parameters even in the case of power failure, greatly improving the convenience of use. In terms of safety performance, this model of pulse tester has a built-in overvoltage protection device, which effectively guarantees the stable operation of the instrument during the test and ensures the safety and reliability of the test work. In addition, it also has the characteristics of easy operation, excellent performance, high reliability and good cost performance. Therefore, it is deeply favored by electrical safety testing laboratories and electronic and electrical appliance manufacturers, and has become their preferred tool for electrical safety testing.
Technical indicators
Technical Projects | parameter |
Comply with standards | ISO 14708-1:2014 & GB 16174.1-2024 20.2 |
Test 1 voltage | 140+70V |
Test 1 Circuit | RLC Circuit |
Damped sine defibrillation waveform | 1.5ms < Tp < 2.5ms; 3ms < Tw50 < 5.5ms |
Output polarity | Positive and negative polarity output can be freely set |
Test 1 Program | First send 3 positive voltage pulse sequences (each pulse interval is 20+2s), then send a negative voltage pulse after an interval of 60+2s |
Test 2 voltage | 140+70V |
Phase switching | Single phase/double phase can be set freely |
Monophasic/Biphasic Truncated Exponential Defibrillation Waveform | 9. 5ms<Td<10.5ms 1μs<tr<5μs 1μs<tf<5μs |
Test 2 Program | First send 3 positive polarity single-phase voltage pulse sequences (each pulse interval is 20+2s), then send a negative polarity voltage pulse after an interval of 60+2s |
Evaluation Criteria | Active implantable medical devices are tested for compliance by checking that they have not been permanently affected and can be reprogrammed to restore settings after completing the steps of Test 1 and Test 2. |
Number of trials | 1 - 999 times |
Pulse generator output mode | Floating output |
Input Power | AC 220V±10%, 50Hz/60Hz |
Dimensions | 680*460*200mm |
weight | About 30 kg |